Why The FDA Cares about Human Factors and So Should You
Human Factors in a Nutshell
Human Factors is an integral part of our everyday! Every time a product, process or system is engineered with the goal of being more efficient for the humans that use it, that’s Human Factors at work. Ultimately, the purpose of Human Factors (HF) is to apply what we know about the strengths and weaknesses of being human to improve the quality of interaction between people and products. Increased quality in this case means reduced human error, increased productivity and increased safety and comfort.
Human Factors is the practice of applying Psychology to Engineering to
increase the quality of interaction between the medical products they develop and the people who use them.
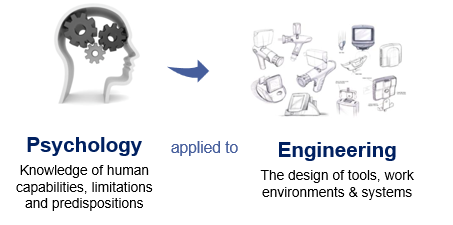
You might also be familiar with the terms “Human Factors Engineering” (HFE) or Usability Engineering (UE). These names are often used interchangeably with “Human Factors” (HF).
Regardless of its name, Human Factors is a critical part of medical device development. Medical device use can often pose risk to clinician and patient users if used improperly. Some of these risks might be minor such as wasted time or inconvenience and some risks can have serious, long-lasting health consequences including death. This is a danger we should all care about and the FDA is leading the way.
What Does this Have to Do with the FDA?
Since 1999, studies have consistently shown the medical errors cause a staggering number of deaths each year. A recent Johns Hopkins study claims more than 250,000 people in the U.S. die annually from medical errors [1]. Other reports claim the numbers to be as high as 440,000 per year [2] and that this number may be even higher since it has been estimated that about 90% of adverse events go unreported! [3] In 2015, another Johns Hopkins study [4] found that medical errors were the third leading cause of death in the U.S., after heart disease and cancer. What is the cause of all these medical errors? This study also found that 15% of medical error deaths are related to difficulties or errors users encounter when using medical device user interfaces (UIs).
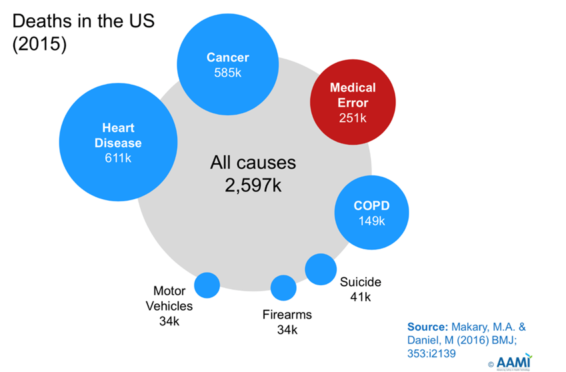
These staggering medical error figures were a wake-up call for the FDA and the healthcare community, in general.
Clearly, patient safety efforts were falling short.
Simply said it appeared that clinicians’ medical knowledge was not enough to conquer the challenges of a hectic and dynamic healthcare environment and to provide consistent and accurate care. Urging clinicians to “try harder” or “be more careful” did not safeguard them against making human errors. Likewise, efforts to improve care solely through education often had minor and fleeting improvements, if any.
The FDA & IEC Response
The FDA was well-aware of the growing medical error crisis and had already updated its regulatory approval guidelines in 2011 in support of a more rigorous human factors process in medical device development during the pre-market phase. In 2016, they renewed their efforts again and published and updated version of their Human Factors Guidance [5] and began to enforce the HF guidelines more rigorously during medical device submission reviews.
These efforts weren’t limited to the US. In 2015, the IEC, an international standards organization, issued 62366-1 [6] which outlined a very similar set of usability engineering expectations for medical device companies as are included in the FDA Guidance. One year later in 2016 62366-2 [7] would also be published which offered additional HF guidance.

These guidance documents assist industry in following appropriate human factors and usability engineering processes to maximize the likelihood that new medical devices will be safe and effective for the intended users, uses and use environments.
The Human Factors Process and Its Benefits
The HF processes described in the FDA Guidance and 62366 are very similar and consist of several steps which are each comprised of several activities. Since the guidances can be a lot to digest, we’ve created a high-level pictogram to communicate the HF process more simply:
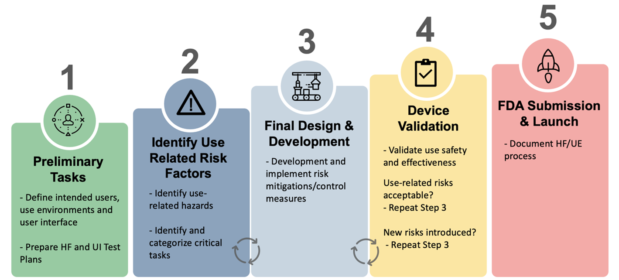
Three key objectives of the HF process in medical device development are to:
- Identify intended users, uses, and use environments,
- Identify potential use-related hazards and risks,
- Minimize these use-related hazards and risks through UI design, labeling and training, and
- Confirm risks have been minimized and that the device is safe and effective to use through usability validation testing
It’s important to understand that the Human Factors process should be applied all elements of the product’s user interface (UI) including:
- Hardware and Software UIs
- Accessories
- Packaging
- Labeling including warnings, instructions, manuals, and package information
- Training materials and training
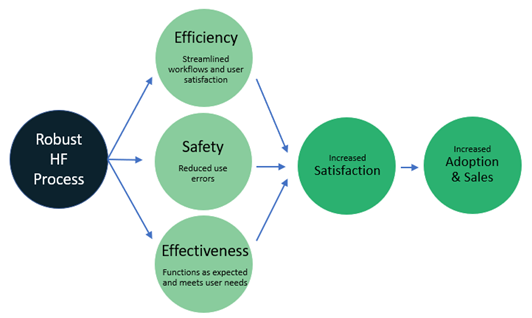
When HF is at work, human safety is prioritized and risk is minimized which helps create a safe, effective device. HF processes also help to minimize usability issues that cause inefficiencies and user frustration, which leads to increase user satisfaction and ultimately, increased adoption and sales.
There are lots of good reasons why you should incorporate HF into your
development process!
Not Convinced Yet? HF Can Help Avoid Submission Deficits and Product Recalls!
We often attend conferences, trainings, and workshops which are led or attended by the FDA’s CDRH Human Factors team. Hearing the latest recommendations and thoughts directly from the FDA’s HF team is invaluable. During her 2021 HFES Workshop “Human Factors Validation of Medical Devices” [8], Janine Purcell, an FDA Human Factors reviewer, brought to the public’s attention that the majority of FDA submissions are returned with Human Factors deficits to correct. In 2019, the FDA reported:
- 95% of 510k Human Factors reviews had HF deficiencies
- 90% of PMA Human Factors reviews had HF deficiencies
- 98% of Q-subs seeking HF protocol review received HF comments (not a bad thing!)
Yikes! That means there’s still more that we all need to do to incorporate Human Factors into our product development processes. Luckily for us, the FDA encourages and supports the pre-submission of the Human Factors Validation Study protocol. As part of this submission, we encourage our clients to include an overview of the full HF process so that the HF Reviewer can spot any deficiencies, ask questions, or provide further guidance before the money and time is spent on the final validation study. We have found this feedback invaluable in our previous pre-subs. By addressing FDA concerns before the final submission, we have saved our client time, money, and effort.
It’s also helpful to be aware of the worst-case scenario for failure to develop a safe and effective product. In another recent talk, this slide caught us by surprise.
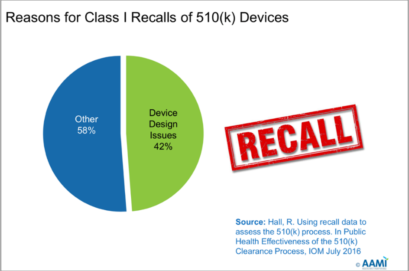
This slide references a study conducted by R Hall in 2016 [9] which researched the reason for Class I 510(k) product recalls by the FDA. It should be noted that Class I products can cause serious harm including death.
50% of Class I 510(k) recalls were caused by issues that could have been
improved by a robust Human Factors process.
Of this 50%, 42% were design issues and 8% were labeling issues – both well within the Human Factors wheelhouse.
Recalls can be devastating to a company. They cost time, money and can be a PR nightmare. Yes, incorporating HF activities does cost time and money during a product’s development process, but the cost/benefit ratio clearly falls in favor of incorporating human factors early in the design process rather than waiting for a failed submission or a recall.
Conclusion
Improper use of a medical device can result in serious injury or even death. Investing in human factors during the design and development process can help identify potential problems and reduce the risk of a device being used incorrectly and causing harm before it ever gets on the market. Products with Human Factors input are not only safer, but are more efficient, effective, and satisfying to use which can lead to higher adoption and sales. Additionally, well-designed, safe products are less likely to have HF submission deficiencies or be recalled.
About the Authors
Shannon Halgren PhD and Agatha Kalinchenko, MD are both senior Human Factors / User Experience Consultants at Sage Research and Design. They and their colleagues at Sage are experienced helping a wide variety of product teams incorporate human factors processes and successfully prepare for their FDA or international submissions. The human factors team follows the FDA Guidance and IEC 62366-1/62366-2 and is skilled at conducting and documenting Use-Related Risk Analyses, analyses of users, uses and use environments, heuristic evaluations/expert reviews, formative and validation usability testing and many other HF activities. We are ready to help with medical devices, drug/biologic products, or combination products.
Sage Research & Design provides HF Process & Design Assessments, Start to Finish HF Services (full package or ala carte), FDA Submission Readiness Evaluations and Human Factors Training. Best of all their small nimble team is flexible, fun to work with, and will quickly demonstrate that they truly care about you and getting your MedTech product through the HF submission process! Contact us to learn more.


REFERENCES
[1] Anderson, J. G., & Abrahamson, K. (2017). Your Health Care May Kill You: Medical Errors. Studies in health technology and informatics, 234, 13–17.
[2] James, John T. (2013). A New, Evidence-based Estimate of Patient Harms Associated with Hospital Care, Journal of Patient Safety,9 (3), 122-128
[3] Classen, D.C., Resar, R., Griffin, F., Federico, F., Frankel,T. (2011). Global trigger tool Shows that adverse events in hospitals may be ten time greater than previously measured. Health Affairs, 30 (4), 581-589.
[4] Makary MA, Daniel M. (2016). Medical error-the third leading cause of death in the US. BMJ, May 3;353:i2139.
[5] FDA 2016. Applying Human Factors and Usability Engineering to Medical Devices: Guidance for Industry and Food and Drug Administration Staff.
[6] ANSI/AAMI, 2015. IEC 62366-1:2015 Medical devices – Part 1: Application of usability engineering to medical devices.
[7] ANSI/AAMI, 2015. IEC 62366-2:2016 Medical devices – Part 2: Guidance on the application of usability engineering to medical devices.
[8] Purcell, J. (2021). Human Factors Validation of Medical Devices. Virtual Presentation, International Symposium on Human Factors and Ergonomics in Health Care.
[9] Hall, R. Using recall data to assess the 510(k) process. In Public Health Effectiveness of the 510(k) Clearance Process, IOM July 2016.

